New coronavirus tests, announced this week, will massively increase diagnostic capacity ahead of winter, delivering fast results that will help to break chains of transmission more quickly.
Millions of ground-breaking rapid coronavirus tests will be rolled out to hospitals, care homes, and labs across the UK to increase testing capacity ahead of winter, the Government revealed this week.
And the tests will enable clinicians and NHS Test and Trace to quickly advise on the best course of action to stop the spread of the virus.
Millions of new rapid coronavirus tests will provide on-the-spot results in under 90 minutes, helping us to break chains of transmission quickly
Two new tests – both able to detect the virus in just 90 minutes – will be made available in the coming weeks.
They will be able to detect both COVID-19 and other winter viruses, such as flu and respiratory syncytial virus (RSV), and do not require a trained health professional to operate them, meaning they can be rolled out in more non-clinical settings.
This will help to further strengthen the coronavirus response this winter, arming both clinicians and NHS Test and Trace with the ability to distinguish between COVID-19 cases, which have specific self-isolation requirements, and other winter viruses.
Health Secretary, Matt Hancock, said: “We’re using the most-innovative technologies available to tackle coronavirus.
“Millions of new rapid coronavirus tests will provide on-the-spot results in under 90 minutes, helping us to break chains of transmission quickly.
“And the fact these tests can detect flu as well as COVID-19 will be hugely beneficial as we head into winter, so patients can follow the right advice to protect themselves and others.”
The tests have been developed by DnaNudge and Oxford Nanopore.
The first test uses DNA to detect the virus and will be rolled out across NHS hospitals from September, with 5,000 DNA machines from DnaNudge providing 5.8 million tests in the coming months.
The technology analyses DNA in nose swabs, providing a positive or negative result for COVID-19 in 90 minutes at the point of care.
The machines will process up to 15 tests on the spot each day without the need for a laboratory.
We are extremely proud to be playing such a pivotal role in supporting the national effort on testing, as this major contract award signifies
Separately, 450,000 90-minute LamPORE swab tests will also be available across adult care setting and laboratories from next week, supplied by Oxford Nanopore.
The new rapid test will be able to process swab and saliva samples to detect the presence of COVID-19 in 60-90 minutes.
It has the same sensitivity as the widely-used PCR swab test, but can be used to process swabs in labs, as well as on-location through ‘pop up’ labs.
And the desktop GridION machine can process up to 15,000 tests a day, while the palm-sized MinION can process up to 2,000 tests a day for deployment in a near-community ‘pop-up’ lab.
In addition, the Government is also signing contracts with more companies to produce more machines for DNA coronavirus testing.
LamPORE has the potential to deliver a highly-effective and, crucially, accessible global testing solution, not only for COVID-19, but for a range of other pathogens
Regius Professor Chris Toumazou, chief executive and co-founder of DnaNudge and a founder of the Institute of Biomedical Engineering at Imperial College London, said: “The DnaNudge team worked with incredible speed and skill during the peak of the pandemic to deliver this highly-accurate, rapid COVID-19 test, which requires absolutely no laboratory or pipettes and can be deployed anywhere with a direct sample-to-result in around just over an hour.
“We have been able to successfully adapt our in-store consumer DNA testing technology – which identifies genetic risks for chronic conditions related to obesity and type 2 diabetes – and validate it for detecting COVID-19 with gold-standard accuracy.
“We are extremely proud to be playing such a pivotal role in supporting the national effort on testing, as this major contract award signifies.
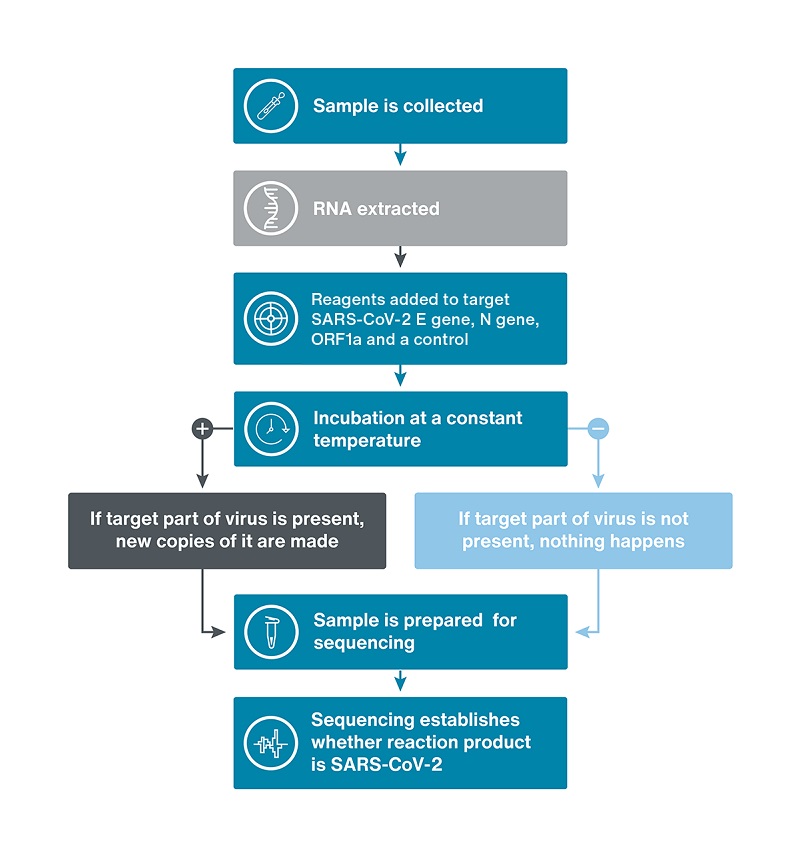
LamPORE is a simple and fast process comprising of amplification, library preparation and sequencing steps to identify whether the SARS-CoV-2 virus is present in a sample
“With the ability to test, not only for COVID-19 but also FluA, FluB and RSV on the same single COVID-19 Nudge cartridge; our multiplex test offers a vital solution to protect the NHS as we head into the flu season.
Gordon Sanghera, chief executive of Oxford Nanopore, adds: “We are honoured to be playing a part in fighting COVID-19 in the UK, and preparing the country for the winter virus season.
“Ever since we founded Oxford Nanopore, our mission has been to create disruptive, high-performance technology that has a profound, positive impact on society.
“LamPORE has the potential to deliver a highly-effective and, crucially, accessible global testing solution, not only for COVID-19, but for a range of other pathogens.
“We are delighted to be working with the UK government to support and empower our communities to effectively manage testing at a national and localised level.”