When Rachel Fallon joined Sky Medical Technology in 2010, the company had less than 10 employees and its focus was ‘OnPulse’ technology, which is at the heart of the geko device.
This device uses electrical charge to stimulate the common peroneal nerve, activating the calf and foot muscle pumps in the lower leg and increasing blood flow in the deep veins, thus assisting the heart in moving blood around the body.
Since joining the company Fallon’s professional challenge has been to turn this technology into a compliant and commercially-viable product.
And the fact that the geko device now has regulatory clearances for use in both the UK and the US, and is backed by clinical research, suggests that this journey has been a success.
However, it has been far from simple.
“To bring a MedTech device to market was a unique challenge that appealed to my experience and interest in medical science and biology,” said Fallon.
“I am a biochemist by training, with experience in molecular and medical science.
“But, having spent a substantial part of my career in biotechnology addressing life science markets, I was keen to take up a new challenge.
My core training in industrial research and development was at Amersham PLC, where I led R&D to develop new technologies and deliver products to market.
Companies need to consider issues such as ensuring continuity of supply as well as building a scalable manufacturing capability that can satisfy the needs of a global market
“The very-broad training I received in more than 20 years has underpinned my career and helped me develop products for commercialisation in several SME businesses.”
Early beginnings
When Fallon joined Sky Medical, the company had prototypes of a product, but little more.
So she quickly identified that any product the company manufactured would need to achieve wide regulatory compliance and be supported by clinical data and commercial success.
“I was given a very wide brief: make the product, prove it works, conduct trials, and move to sales”, she said.
Productisation involved a wide range of functions including R&D, regulatory affairs, manufacturing and clinical affairs, as well as real-world clinical and economic evidence of success.
And Fallon is clear about the scale and breadth of the challenge to bring a MedTech product to market.
“Our first task was to identify the unmet clinical need and the market requirements for both product development and generating clinical data.
“Right from the start, the design process needed to balance the patient requirements with the challenges of manufacturing a robust and reliable product.
“In MedTech there is the further issue of complying with medical device regulations, as well as other applicable regulations, for example around electrical safety and biocompatibility.”
The initial challenge was to develop a disposable product with a relatively-low cost of goods and a simple, robust manufacturing process.
“Companies need to consider issues such as ensuring continuity of supply as well as building a scalable manufacturing capability that can satisfy the needs of a global market,” advises Fallon.
“This is particularly important where patients are relying on a device to address medical issues and where an interruption of supply could negatively impact a patient’s wellbeing.”
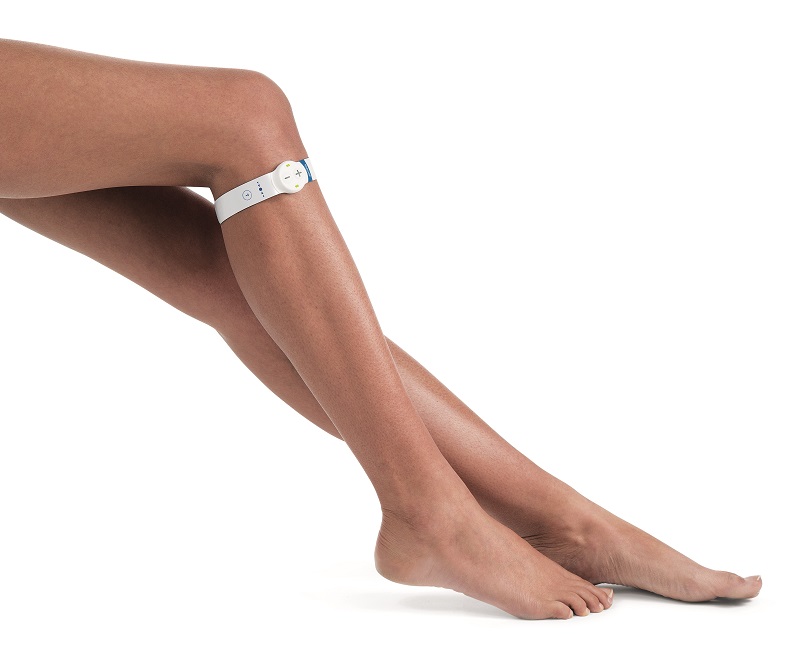
Sky Medical Technology's geko device now has regulatory clearance in the UK and US
A focus in clinical application
Sky Medical faced an important early decision around the focus for the product’s position in the market.
“The ability to increase blood circulation in patients with limited mobility has the potential to deliver positive medical outcomes for a wide range of medical issues when used in the hospital or home setting,” said Fallon.
It is important to remember that instructions on how to use the device need to comply with specific regulation in every country where it is being used, which will differ and are subject to change
“I think at one point there were more than 30 potential applications to be considered.
“However, we need to create clinical and economic evidence to support every different clinical indication where increasing blood flow is beneficial to the patient.
“As a business, we also need to prioritise which applications to target according to our resources.”
Ultimately Sky Medical focussed on three areas: the prevention of venous thrombosis (VTE); the prevention and treatment of oedema (swelling); and wound therapy applications (hard-to-heal leg ulcers).
The total addressable market opportunity for the geko device in each of these areas is £202m, £282m, and £603m respectively.
While Sky Medical believed that the geko device would deliver in each of these areas, new clinical evidence was needed to support each application.
“Bringing new MedTech to market is obviously challenging – and it should be,” says Fallon.
“It’s clearly a huge advantage having what we describe as a ‘platform’ technology – one that is applicable to a variety and range of medical conditions.
“However, every application should be considered as its own separate product.
“For example, the clinical evidence that supports VTE prophylaxis is not applicable for the woundcare market.
“Furthermore, different product variants are needed depending on how they are used.”
Regulatory hurdles
These efforts were further complicated by the regulatory environment around medical devices.
The geko device is the first bioelectronic product of its kind to receive both NICE approval and FDA clearance for VTE prevention across all patients including non-surgical patients.
Despite this, there is a need to obtain approval for new indications for use by territory while maintaining existing clearances as the regulatory landscape changes globally.
The key is keeping an eye on the lead time for product components and device assembly together with a rolling sales forecast for product by territory
“Clearly you have to consider how the patient will use the product and to make it as simple as possible for whoever is administering the device,” said Fallon.
“But it is also important to remember that instructions on how to use the device need to comply with specific regulation in every country where it is being used, which will differ and are subject to change.
“I believe we have translated and localised instructions more than 20 times to ensure compliance with the major regulatory bodies across the UK, the European Union, and the Americas.”
Manufacturing smart
Regulatory compliance has an impact on manufacturing – not only in terms of ensuring a reliable and compliant product, but also managing specific stock levels for specific markets.
Excess stock can be a significant cash drain for any start-up business, but in MedTech there is further friction caused by ensuring there is always a steady and reliable supply of devices.
And this is complicated because stock cannot simply be reallocated from country to country without changing packaging and instructions to reflect different regulatory jurisdictions.
Demand management is therefore essential.
Fallon said: “We ensure there is close and regular communication between the commercial team, finance, and manufacturing.
“The key is keeping an eye on the lead time for product components and device assembly together with a rolling sales forecast for product by territory.
“In manufacturing, our aim is to be as scalable as possible, and I bring people representing R&D and the operational functions together on a weekly basis to try to ensure there are no surprises.
“To meet the expectations of customers means considering all relevant issues in order to define the next steps.
“We have developed a bespoke automated assembly line which we commissioned in 2020. The line has increased our production capacity by 500% and it is ultimately scalable to three million devices per line, per annum.
Having really-smart, bright people in the business is a pre-requisite to creating an innovation culture
“It has also given us the ability to scale the assembly process across multiple assembly lines and then potentially replicate in different geographies in the future.”
A recent example was an urgent request from the NHS Nightingale hospitals during the COVID-19 pandemic.
Having identified blood clotting as a potential complication from Coronavirus, the NHS potentially needed thousands of geko devices at short notice.
Fallon said: “We spent a good part of the Easter weekend in 2020 working out what we could supply and by when.
“We looked at the supply chain for key components, shift patterns within the factory, and other factors to create an accurate forecast that we could be certain to fulfil.”
Sky Medical has also invested significantly in building and continuously improving its Quality Management System to ensure compliance with medical device regulations including ISO13485 and 21 CFR.
By participating in the Medical Device Single Audit Program (MDSAP) Sky Medical has gained access to multiple geographies including the Americas and Australia through an efficient, single audit process.
But Fallon is quick to emphasise the importance of quality critical elements of the manufacturing process.
“We’ve invested time and money into our manufacturing processes”, she said.
“We specify our components and audit our approved suppliers in order to achieve a reliable, predictable manufacturing process.
It’s important to give experts the environment and resource they need to flourish, but, equally, to share the business context for their role and what they are striving to achieve
“Together this offers reassurance that people can trust device quality.”
Open communication
In many ways, Fallon’s role at Sky Medical is primarily that of a communicator.
She describes her role as ‘bringing the right people together to ensure they can make better, more-informed decisions for the business’.
She adds: “I’m fortunate to have a team of experts around me in many areas: manufacturing; regulation; design; research and development; and clinical trials.
“My job is not to know their role better than them, but to extract and share the relevant information that other parts of the business need to know – be that commercial, marketing, or operations.”
She sees this role as creating a culture where innovation can flourish within a focused delivery plan.
“Having really-smart, bright people in the business is a pre-requisite to creating an innovation culture,” she said.
“But it is also incredibly important to engage intelligently in the conversations that are taking place.
“It’s important to give experts the environment and resource they need to flourish, but, equally, to share the business context for their role and what they are striving to achieve.
“People need to understand the importance of their role to the wider business objectives.
“This is essentially, at heart, a communications challenge. People need to know not only what they are doing, but why they are doing it.
“We aim to create an environment where great ideas can flourish; a safe-but-accountable space for innovation.”




